Nuclear Reactor Location Map
Protect Your Family
U.S. Nuclear Reactor Information
- Zip Code Locator Map of U.S. Nuclear Reactors
- Map of U.S. Research Nuclear Reactors
- Chernobyl and radioactive iodine
- The NRC’s Consideration of Potassium Iodide in Emergency Planning
- American Thyroid Association: Nuclear Radiation and the Thyroid (PDF)
- Detailed list of U.S. power-generating nuclear reactor locations
- Detailed list of U.S. research nuclear reactor locations
- Canada’s nuclear reactors and uranium mines
Find a U.S. Nuclear Reactor Location
Live beyond that? The American Thyroid Association recommends potassium iodide be made available to EVERYONE else in the country, regardless of your distance from a nuclear reactor.
Why? Radioactive iodine released from the Chernobyl and Fukushima reactors and nuclear bomb testing traveled hundreds of miles from the source, causing over 200,000 cases of thyroid cancer.
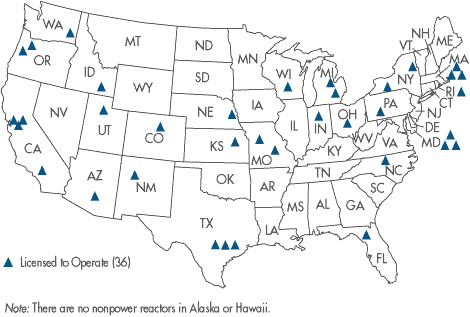
Map of U.S. Research Nuclear Reactors
There are 36 non-power nuclear reactors currently operating in the U.S. These smaller nuclear reactors, which do not generate power, are in locations such as universities and other organizations where research, testing or training is conducted. Research nuclear reactors produce radioisotopes for nuclear medicine, are used to train people in the nuclear sciences and are used as a laboratory tool, as a producer of byproduct material or as a source of radiation for experiments. Here is a detailed list of U.S. research reactors.
Chernobyl and radioactive iodine

Potassium Iodide was distributed in the immediate areas surrounding the Chernobyl Nuclear Station, including the Pripyat region where most of the workers lived. Controlled by the weather conditions at the time, the radioactive plume touched down again and again in numerous populated areas as far out as 500 km (over 300 miles). Below are facts as extracted from official accounts and government publications.
Chernobyl: What’s been learned, What’s been ignored
Chernobyl and thyroid cancer
97% of all thyroid cancer cases attributed to radioactive iodine from Chernobyl occurred in people who lived 31-310 miles from the accident site. (Due to the distribution of Potassium Iodide in the areas nearer the accident site including the Pripyat region)
– Statistics derived from U.S. Nuclear Regulatory Commission Report NUREG-1633
“Evidence of a marked excess of thyroid cancer in children exposed to the fallout from the Chernobyl accident has been established. In the most affected areas of Belarus, the yearly incidence has risen close to 100 per million children, which is more than 100-fold compared to the situation before the accident. It is now generally accepted that this excess has resulted from exposure to the radioactive iodine released in the accident”.
“Most of the children that have developed thyroid cancer were exposed to an estimated dose to the thyroid of less than 300 mGy. There has been an excess thyroid cancer incidence even in areas where the mean dose to the thyroid in children was estimated at 50-100 mGy. The increase in incidence has been documented up to 500 km [310 miles] from the accident site. This is understandable in terms of the wide area affected by radioiodine and therefore the large number of children exposed”.
“The Chernobyl accident has thus demonstrated that significant doses from radioactive iodine can occur hundreds of kilometers from the site, beyond emergency planning zones. A sharp distinction in the requirements for stable iodine prophylaxis based on distance from the accident site cannot be made”.
– World Health Organization, Geneva,
1999 Guidelines for Iodine Prophylaxis following Nuclear Accidents
“The number of people with thyroid cancer began to increase about five years after the accident. This number continues to rise. Today, over 11,000 cases of childhood thyroid cancer due to Chernobyl have been reported, with the number not expected to peak until 2010. The World Health Organization’s International Thyroid Project has found evidence that even relatively low levels of radiation exposure may result in underactive thyroid syndrome, also known as hypothyroidism”.
– United Nations Office for the Coordination of Humanitarian Affairs- 2000
“Except for thyroid cancer, there has been no confirmed increase in the rates of other cancers, including leukemia, among the first responders, cleanup workers and military personnel in the contaminated zones following the accident, or the public, that have attributed to releases from the accident”
– U.S. Nuclear Regulatory Commission NUREG-1633
“The vast majority of the thyroid cancers were diagnosed among those living more than 50 km (31 miles) from the site”. – U.S. Nuclear Regulatory Commission NUREG-1633
“Stable iodine could also be used as prophylaxis against ingested radioactive iodine from contaminated food. However, because the risk of exposure from ingestion of iodine remain for a longer time, iodine prophylaxis will also be required for a longer period of time“
– World Health Organization, Geneva,
1999 Guidelines for Iodine Prophylaxis following Nuclear Accident.
 |
The NRC’s Consideration of Potassium Iodide in Emergency Planning(information provided by the U.S. Nuclear Regulatory Commission) |
- Eligibility for Obtaining Potassium Iodide
- Process for Obtaining Potassium Iodide
- Distribution of Liquid Pediatric KI
- Regulations and Guidance
- Current Status on KI Distribution
- Role of Reactor Licensees
- Distribution of KI Within 20-Mile Radius of Nuclear Power Plants
Remarks on the need for potassium iodide made by Commission Chairman Nils Diaz of the Nuclear Regulatory Commission …”use of potassium iodide pills would have significantly reduced the incidence of thyroid cancer [during Chernobyl]”.
The Nuclear Regulatory Commission has revised a section of its emergency preparedness regulations. The revised rule requires that States* with a population within the 10-mile emergency planning zone (EPZ) of commercial nuclear power plants consider including potassium iodide as a protective measure for the general public to supplement sheltering and evacuation in the unlikely event of a severe nuclear power plant accident.
The final rule amends 10 CFR 50.47(b)(10). The NRC published the rule change in the Federal Register (Volume 66, Number 13, page 5427) on January 19, 2001. The change became effective April 19, 2001.
Along with this rule change, the NRC is providing funding for a supply of potassium iodide for a State that chooses to incorporate potassium iodide for the general public into their emergency plans. After funding the initial supply of potassium iodide, the Commission may consider extending this program to fund replenishment supplies, but has made no commitments in this regard.
Potassium iodide is a salt, similar to table salt. Its chemical symbol is KI. It is routinely added to table salt to make it “iodized.” Potassium iodide, if taken within the appropriate time and at the appropriate dosage, blocks the thyroid gland’s uptake of radioactive iodine and thus reduces the risk of thyroid cancers and other diseases that might otherwise be caused by thyroid uptake of radioactive iodine that could be dispersed in a severe reactor accident.
The NRC and the Federal Emergency Management Agency (FEMA) are the two Federal agencies responsible for evaluating emergency preparedness at and around nuclear power plants. The NRC is responsible for assessing the adequacy of onsite emergency plans developed by the utility, while FEMA is responsible for assessing the adequacy of offsite emergency planning. The NRC relies on FEMA’s findings in determining that there is reasonable assurance that adequate protective measures can and will be taken in the event of a radiological emergency.
The Food and Drug Administration (FDA) is the definitive medical authority in the United States on the use of potassium iodide.
*When used in this Web site, State includes Native American governments.
Eligibility for Obtaining Potassium Iodide
This rule applies to States and Tribal governments with nuclear power plants within their borders, with populations within the 10-mile EPZ, and local governments designated by States to request potassium iodide funding.
The Commission believes the final rule, together with the Commission’s decision to provide funding for the purchase of a State’s supply of potassium iodide, strikes a proper balance between encouraging (but not requiring) the offsite authorities to take advantage of the benefits of potassium iodide and acknowledging the offsite authorities’ role in such matters. By requiring consideration of the use of potassium iodide, the Commission recognizes the important role of States and local governments in matters of emergency planning.
Process for Obtaining Potassium Iodide
On December 20, 2001, the NRC sent letters to the 34 States with populations within the 10-mile EPZ of nuclear reactors. This letter discusses the NRC program to provide potassium iodide to States and includes, as attachments: the NRC Statement of Consideration in support of the final rule; the FDA final guidelines on use of potassium iodide; and FEMA guidelines on incorporating potassium iodide into emergency response plans, as well as the NRC disclaimer .
The revised Federal Policy on Use of Potassium Iodide was also provided to the States.
The Office of Public Affairs issued a press release on 12/20/01, to announce the NRC’s potassium iodide program.
Distribution of Liquid Pediatric KI
On January 12, 2005, the FDA approved the ThyroShield oral solution of 65mg/mL dose for use in children. On November 10, 2005, the NRC, in cooperation with the Department of Health and Human Services, sent letters to the States announcing the availability of 51 million doses of ThyroShield liquid pediatric KI for States with populations within the 10 mile EP
Process for Replenishment of Existing Potassium Iodide Stockpiles
On October 26, 2006, the NRC sent letters to the 21 States that have received 130 and/or 65 mg potassium iodide tablets through the NRC’s initial KI offer. This letter discusses the NRC’s decision to replace expiring stockpiles of potassium iodide on a one-time basis as well as instructions for obtaining replacement KI stockpiles. The letter includes, as attachments, the FDA final guidelines on use of potassium iodide; the NRC Statement of Consideration in support of the final rule; the NRC disclaimer; and Staff Requirements – SECY-06-0142 – Options and Recommendations for Replenishing Expired Potassium Iodide (KI).
Shelf Life Extension and Replenishment of Existing Potassium Iodide Stockpiles
NRC received numerous inquiries from States regarding the possibility of extending the shelf-life of the KI tablets in light of the approval by FDA to the manufacturers for product shelf-life extension. The manufacturer (RECIP) of the 65-mg tablet issued an extension of the product by lot number. The manufacturer (Anbex) of the 130 mg tablets in a correspondence from its legal counsel to the NRC indicated that there are no significant differences in the formulation, manufacturing process, or packaging materials for the existing lots as compared to current lots. The FDA in a letter to NRC stated that “It would be considered to be scientifically sound if the lots having the expiration date extended had no significant difference in formulation, manufacturing process or packaging materials from current lots. The letter …to NRC from counsel to Anbex, Inc. dated January 23, 2007 indicates that this is the case.” In a letter to the States, the NRC transmitted the FDA correspondence on shelf-life extension and Commission direction to Staff (SRM-COMSECY-07002 ) on full and partial replenishment of KI, and announced the availability of additional pediatric liquid KI.
States that choose to extend their stockpiles must document the lot number(s) of the KI product, the current expiration date, and the extended expiration date. This document should be available for review by representatives from DHS/FEMA Radiological Preparedness Program during the routine evaluations of offsite emergency preparedness.
Regulations and Guidance
The NRC final rule on the Consideration of Potassium Iodide in Emergency Plans was published in the Federal Register on January 19, 2001. This rule became effective April 19, 2001. The FDA final guidance on Potassium Iodide as a Thyroid Blocking Agent in Radiation Emergencies was published in December 2001. The Federal Emergency Management Agency published the revised Federal Policy on the Use of Potassium Iodide in January 2002.
Current Status on KI Distribution
Twenty states (Massachusetts, Connecticut, Maryland, Vermont, Delaware, Florida, Alabama, Arizona, New York, New Jersey, North Carolina, South Carolina, Pennsylvania, California, Ohio, Virginia, Mississippi, West Virginia, New Hampshire, and Tennessee) have requested and/or received potassium iodide tablets.
Role of Reactor Licensees
The Commission notes that this rule will introduce another element in the context of emergency planning requirements for which licensees are ultimately responsible. Licensees have the obligation to confirm that offsite authorities have considered the use of potassium iodide as a supplemental protective action for the general public. It will also require the licensees to use this information in developing Protective Action Recommendations for offsite agencies.
Distribution of KI Within 20-Mile Radius of Nuclear Power Plants
Section 127 of the Public Health Security and Bioterrorism Preparedness and Response Act of 2002 (the Bioterrorism Act) requires State and local governments through the national KI stockpile to distribute KI tablets to population within 20 miles of a nuclear power plant. The Bioterrorism Act also directed the National Academy of Sciences (NAS) to study the expanded distribution of potassium iodide and report back to the President on the best distribution methods to accomplish such an expanded distribution. The NAS published this study in January 2004.
On August 29, 2005, the Department of Health and Human Services (HHS) published draft guidelines for State, local, and tribal governments, for the expanded distribution, stockpiling, and utilization of KI in the event of a radioactive iodine release from a commercial nuclear power plant incident. On September 2, 2005, HHS issued a correction to add deadline for receiving public comments. The NRC provided comments to the draft guidelines on November 1, 2005.
On July 3, 2007, President Bush delegated his authority to invoke the waiver provision in Section 127 to his science advisor, Dr. Marbuger, Director of the Office of Science and Technology Policy at the White House. In a January 22, 2008 memo, Dr. Marburger announced his decision to invoke the Section 127(f) waiver. A technical paper on the issue of KI was prepared by the Federal Radiological Protection Coordinating Committee KI Sub-Committee at the request of Dr. Marburger as part of his determination process.